Portions of the text below have been excerpted from the following NALMS publications:
Carlson, R.E. and J. Simpson. 1996. A Coordinator’s Guide to Volunteer Lake Monitoring Methods. North American Lake Management Society. 96 pp.
Introduction
Phosphorus is probably the most studied plant nutrient in freshwater aquatic sciences. It is often found to be (and more often inferred as) the nutrient that limits the growth and biomass of algae in lakes and reservoirs. Whether this nutrient is as universally limiting as once believed is debatable, but certainly there is substantial evidence of its importance in many lakes. Numerous correlations and regressions have been constructed linking phosphorus, especially total phosphorus, with variables such as algal chlorophyll, algal weight, and productivity. Because of its possible importance in limiting the growth and biomass of algae and because of the numerous empirical models available for phosphorus, it is an important addition to the list of variables to be measured in volunteer programs.
Forms of Phosphorus
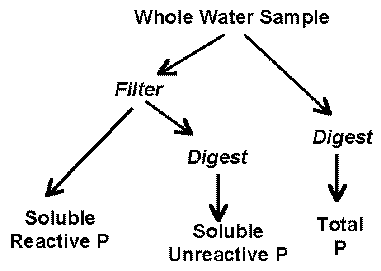
Phosphorus in natural waters is divided into three component parts: soluble reactive phosphorus (SRP), soluble unreactive or soluble organic phosphorus (SUP) and particulate phosphorus (PP) (Rigler 1973). The sum of SRP and SUP is called soluble phosphorus (SP), and the sum of all phosphorus components is termed total phosphorus (TP). Soluble and particulate phosphorus are differentiated by whether or not they pass through a 0.45 micron membrane filter. The phosphorus fractions are illustrated below:
Soluble Reactive Phosphorus (SRP)
This phosphorus fraction should consist largely of the inorganic orthophosphate (PO4) form of phosphorus. Orthophosphate is the phosphorus form that is directly taken up by algae, and the concentration of this fraction constitutes an index of the amount of phosphorus immediately available for algal growth.
In phosphorus limited situations, the concentration of this form should be very low to undetectable (<5 µg/L). As concentrations of orthophosphate (as reflected in the SRP fraction) increase, it can be inferred that phosphorus is either not needed by the algae or that it is being supplied at rates faster than it can be taken up by the biota. Measurement of SRP can be used as an indicator, albeit a potentially inaccurate one, of the degree of phosphorus limitation of the algae.
At one time SRP was called “dissolved inorganic phosphorus.” This terminology was changed to “soluble reactive phosphorus” (Rigler 1964; Strickland and Parsons 1965) to reflect a more realistic interpretation of what forms of phosphorus were found in this fraction. The terms “soluble” and “reactive” were chosen instead because this form of filtered phosphorus was neither necessarily dissolved nor necessarily inorganic.
The term “reactive” is used to indicate that the phosphorus in the SRP fraction is not solely inorganic phosphorus, but could include any form of phosphorus, including some organic forms, that react with the reagents. Some organic forms apparently do hydrolyze and react under the conditions of this test, while some forms of inorganic phosphorus (polyphosphates), in fact, do not react. There is a continuing debate as to what extent SRP represents solely the ortho form of phosphorus or is biologically available (Nürnberg and Peters 1984). The actual composition of SRP probably varies with the nature of the water body.
The “soluble” fraction does not necessarily contain only dissolved phosphorus forms: the phosphorus containing material in the soluble fraction is dependent on the porosity and characteristics of the filter used. Typically, a 0.45 micron cellulose (Millipore) filter is used as a standard. This filter excludes most particulates, but colloidal phosphorus may be present in the filtered fraction.
Some analytical protocols use glass fiber filters instead of a membrane filter. Using a glass filter increases the amount of particulate material that passes through the filter and therefore increases the amount in the “soluble” fraction. Small particulates, including very small algae and bacteria will be present in the filtered sample. Whether or not they become represented as SRP will depend on the extent that they react with the reagents. Glass fiber filters are less costly than the membrane filters, and, more important, are used in a number of other tests such as chlorophyll, particulate carbon, and suspended solids. There is no consensus as to whether the necessity of separating the soluble fraction from all possible particulate forms is more important than analytical consistency between variables.
Some researchers in phosphorus dynamics use Nucleopore™ filters instead of either membrane or glass fiber filters. These filters partition the water into size fractions more accurately than do membrane or glass fiber filters, and therefore allow much better separation of size related phosphorus forms. Nucleopore filters also do not contain phosphorus, which is a contaminant in membrane filters (Peters, personal communication). However, these filters are more expensive than either membrane or the glass fiber filters, and the increased control of size may not be important to a general monitoring effort.
Soluble Unreactive Phosphorus (SUP)
This phosphorus fraction contains filterable phosphorus forms that do not react with the phosphorus reagents under the time and conditions of the test. It is measured as the difference between soluble phosphorus (SP) and SRP. The compounds in the SUP fraction are organic forms of phosphorus and chains of inorganic phosphorus molecules termed polyphosphates. The size of this fraction relative to the other phosphorus fractions is highly dependent on the type of filter used to separate the soluble from particulate fractions.
A number of organic phosphorus molecules have been identified, but two main classes seem to predominate in natural waters. The first are low molecular weight compounds, apparently derived from algal and bacterial metabolism, which release orthophosphate upon treatment with alkaline phosphatase. These compounds do not react with the phosphorus reagents without prior digestion (Franko and Heath 1979). The second are colored, large molecular weight compounds, perhaps phosphorus bound in humic complexes, which release orthophosphate in the presence of ultraviolet light.
These compounds may (Downes and Paerl 1978) or may not (Franko and Heath 1979) react without prior digestion and could be measured as SRP. These organic forms apparently form a pool of phosphorus for algal and bacterial growth, but they must be first converted to orthophosphate, either by enzymes or by UV light, before they are available for uptake by the biota. Although the phosphorus in the pool appears to be highly dynamic, the total amount of phosphorus in this fraction seems to be fairly stable seasonally in lakes with long residence times (Rigler 1964). However, changes in the pool might signal shifts in either the availability of this phosphorus form to algae or bacteria or shifts in the biotic community’s ability to use this form, or more likely, runoff variability into the lake.
Soluble Phosphorus (SP)
This form is measured after the digestion of the filtrate and should contain all filterable forms of phosphorus, both organic and inorganic that are converted to orthophosphate by the digestion process. However, the amount of phosphorus in this filterable pool is highly dependent on the filter used. The larger the effective pore size of the filter, the more particulate material that will pass through the filter, be digested, and be considered “soluble.”
Rigler (1964) estimated the percent of phosphorus that would be considered part of the soluble phosphorus pool of three lakes, using 7 different separation techniques and filter sizes. Particulates were removed using a Foerst centrifuge, or by passing the water through 3 layers of Whatman #44 paper filters, or through 5.0, 1.2, 0.45, 0.22, and 0.1 micron Millipore filters. Considerable differences in the percent soluble phosphorus were found as the pore size decreased.
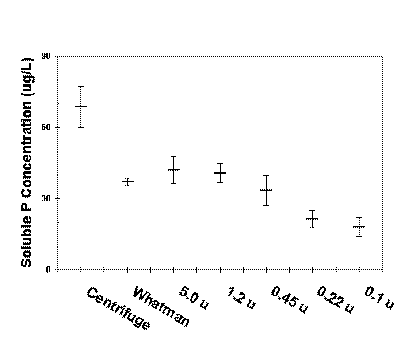 |
The effect of particle separation method or filter pore size on the estimate amount of soluble phosphorus in lakewater (mean plus or minus standard error). Data from Rigler 1964. |
Deviation from the 0.45 micron membrane standard will have a significant effect on the soluble phosphorus concentration, and, because it is calculated as the difference between SP and SRP, soluble organic phosphorus will be affected as well. SRP may or may not be affected, depending on the extent to which phosphorus in these particulates are released during the analytical procedure.
Particulate Phosphorus
This fraction of phosphorus contains all material, inorganic and organic, particulate and colloidal, that was captured on the filter. Typically, particulate forms will contain bacteria, algae, detritus, and inorganic particulates such as clays, smaller zooplankton, and occasionally, larger zooplankton, sediments, or large plant material.
Particulate phosphorus can be measured either by filtering a known volume of water through a membrane filter and then digesting the filter, or it can be obtained by subtraction of total soluble phosphorus (SP) from the total phosphorus concentration (TP). The filtration method allows the analyst to concentrate samples from low particulate waters, thereby increasing the sensitivity of the test, but it also increases the probability that large particles, such as zooplankton will be captured as well. Caution must be taken so that adequate oxidant is present to completely digest the sample.
Large zooplankton, suspended sediments, or large plant remains may be captured on the filter. These latter particulates are generally considered to be contaminants rather than normally occurring portions of the fraction. Although suspended sediments, especially if stirred up during the sampling process or stray leaf or plant remains should be considered a contaminant, zooplankton in the sample present an interpretational problem, especially in low phosphorus situations. Zooplankton can significantly increase the phosphorus concentration in the water sample. Chamberlin (1968), as cited in Rigler (1973), found that a single individual of the zooplankter, Daphnia, containing 0.19 g P, would increase the total P in a 50 ml water sample by 4 µg/L. Prepas and Rigler (1982) found that one phantom midge larva (Chaoborus) can add 100 µg/L to a 50 ml sample.
Zooplankton and other large particulates cause a problem to particulate and total phosphorus analyses because they are probably inadequately sampled, and therefore give an inaccurate phosphorus estimation. If particulate P is determined by subtraction, zooplankton are probably not sampled accurately or randomly in a 10 or 50 ml aliquot used in the total phosphorus determination. The presence or absence of the few zooplankton that may be captured would cause a significant overestimate or underestimate of the limnetic phosphorus pool if zooplankton are considered part of that pool. Although it might seem that filtering and digesting the filter would increase the precision of the particulate measurement, its accuracy is still in doubt because the tools used for collecting total phosphorus from the lake (water samplers or tube samplers) will generally under sample zooplankton populations. By either method, the limnetic phosphorus pool will be inaccurately measured.
Certainly, the larger size fraction of the limnetic phosphorus pool (zooplankton and fish) are generally ignored in most studies, although they may make up a sizeable fraction of the total phosphorus in the water. For example, Prepas and Rigler (1982) found that the phosphorus fraction greater than 250 microns (which might contain larger algae as well as zooplankton) constituted between 14 and 28% of the total phosphorus in the epilimnion on an Ontario lake. Fish can also constitute a sizable proportion of the total phosphorus in a lake. Kitchell et al. (1975) reported that zooplankton constituted 18% and fish, 74%, of the total pelagic zone phosphorus in Lake Wingra, Wisconsin. Generally these larger size fractions do not interfere with the use of total phosphorus in empirical models because the data used in model construction probably ignored these size fractions as well. However, if the data were used to estimate lake phosphorus content for a nutrient budget, then it is possible that the content could be seriously underestimated without consideration of the larger fractions.
The ideal would be to have a size fractionated phosphorus, where phosphorus would be sampled by methods appropriate for each size fraction. In the absence of the ideal, which is probably the case of volunteer programs, particulate phosphorus is probably best measured by subtraction from total phosphorus rather than filtration. In this case, the particulate value will be influenced by the pore size of the filter used. Volunteers could be cautioned to try to exclude large particles such as leaves.
Total Phosphorus
This form incorporates the total of all filterable and particulate phosphorus forms mentioned above. It is probably the most often analyzed fraction of phosphorus because it is used in a wide variety of empirical models relating phosphorus to a wide variety of limnological variables (Peters 1986); and the link between phosphorus loading estimates and phosphorus content in the lake.
Considering the wide variety of materials that might constitute “total phosphorus,” it is remarkable that total phosphorus correlates well with any other single variable, especially an algal variable such as chlorophyll. The chlorophyll molecule itself does not even contain phosphorus. For such correlation to be strong, chlorophyll must be related to phosphorus containing compounds in algal cells. Variations in TP chlorophyll relationships come, in part, from variations in the amount of chlorophyll generated per unit of total phosphorus in algal cells. As cells vary in their phosphorus or chlorophyll content, the TP/chlorophyll ratio will vary. All other phosphorus forms found in the water must be constant, negligible, or at least change as a function of chlorophyll.
For example, SRP is generally low in phosphorus limited situations while SUP may be relatively constant. Increases in SRP or variations in SUP would adversely affect chlorophyll phosphorus relationships. Forms of particulate phosphorus, other than algal phosphorus, must also be negligible, constant, or change as a function of chlorophyll. Chlorophyll would be expected to relate best to total phosphorus in situations where there are negligible amounts of clays, suspended sediments, and detritus, where phosphorus limits algal biomass, and in natural lakes that have sufficient depth that bottom sediments are not resuspended.
“Total” phosphorus is largely defined on the basis of how much phosphorus in its various forms will be oxidized into orthophosphate by a specific oxidant. Different analytical tests used for the digestion and analysis of phosphorus may change the amount of phosphorus reported. It is important to remember that all these phosphorus designations are functionally defined, and the coordinator must be very cautious about modifying phosphorus analysis procedures. It is possible that some modifications would alter the results significantly and therefore limit the use of the data. It is also important to remember that all of the empirical equations are based on certain methodologies (hopefully, the same ones). Deviations from these methods could introduce error in these empirical relationships (Griesbach and Peters 1991).
Phosphorus Collection and Analysis
Sampling and Preservation
Although field kits that measure phosphorus potentially could be used by volunteers, the limits of detection of the kits that use visual comparators are too high to be useful in lake monitoring programs. A sensitive spectrophotometer is necessary to measure the phosphorus levels found in most lake situations. Because of this, volunteers will most often be asked to collect samples for later analysis in a laboratory. Prime concerns should be that the volunteer collects the sample correctly and that the sample is stored in a manner that will allow subsequent analysis with minimal changes in either the absolute concentration or phosphorus form.
Collection of the water sample for subsequent analysis can be done with any of the sampling techniques discussed in the earlier chapter on sampling techniques. Surface (0.5 m) samples are usually used in trophic state determinations, but see the chapter on sampling for a discussion of alternative sampling procedures. Whatever the technique chosen, the phosphorus sample should be taken from the same water sample that is used for chlorophyll. Hypolimnetic phosphorus samples can also be taken, but as the Wisconsin program discovered, it is difficult to obtain a near sediment sample without disturbing the sediments. Care should be taken that the sampler is clean, without any dirt or material from earlier excursions clinging to the inside surface. Several rinsings with lake water prior to use may be beneficial.
Once collected, the water should be poured into a pre-cleaned sample container. It would be best that this sample container be cleaned and supplied by the program rather than relying on the volunteer to supply or to clean the container. Cleaning should consist of washing the containers in phosphorus free detergent. (usually dish washing detergent, not dishwasher detergent, is phosphorus free), several tap water rinses, a rinse in dilute HCl (Standard Methods APHA 1989, recommends hot dilute HCl) and then several rinses in distilled water. If care is not taken in the cleaning of the containers, subsequent care becomes meaningless. Standard Methods (APHA 1989) also recommends that plastic jars not be used unless the sample is frozen because the phosphorus may be adsorbed onto the walls of the plastic container. If the sample is to be poured from the container in the laboratory prior to analysis, the sample should be preserved prior to storage to prevent any transformations (uptake by algae or bacteria) or sorption to the walls of the container during storage. If only total phosphorus will be measured, then the sample can be preserved with one ml of HCl per liter or by freezing the sample.
An alternative method used in several Midwest program is to measure and pour an exact amount of the sample into a pre-cleaned borosilicate bottle. Later, digestion is done in this same container. The assumption is that preservation is not necessary because any material adsorbed onto the side of the container will be stripped off during the digestion process.
Griesbach and Peters (1991) found that TP in unpreserved, unfiltered samples stored frozen was stable for up to 12 months, if stored in the tubes in which they would eventually be analyzed. At room temperature, samples could safely be stored for a month, again, if stored in the tubes in which they would be analyzed. If other forms are to be analyzed, filtration prior to preservation seems to yield the best results. If clean filtration equipment can be assured, there is no reason that the volunteers could not do the filtration to differentiate between soluble and particulate phosphorus. If glass fiber filters were used, then the filtrate obtained from the chlorophyll filtration could be used for the soluble phosphorus fraction. The problem of volunteer obtained filtered samples is that of preservation. Standard Methods (AHPA 1989) recommends that the sample should be immediately frozen at or below 10 °C or preserved with HgCl2. Mercuric chloride is extremely hazardous and should never be given to volunteers.
Griesbach and Peters (1991) found that filtered samples kept at room temperature could be analyzed for SRP and SP within a week, if stored in the tubes in which they would be eventually analyzed. They did not recommend freezing samples for analysis of the phosphorus fractions. Others report maximum holding times for SRP analysis from less than 24 hours to a week.
Limits of Detection
Although a number of analytical tests exist for the measurement of phosphorus, the ascorbic acid method described in Standard Methods (AHPA 1989) is probably the most commonly used test. In this test, the molybdate reagent reacts with orthophosphate producing phosphomolybdic acid, which forms the colored molybdenum blue upon reduction with ascorbic acid. While the compound appears blue, the peak absorbance at 885 nm is in the infrared region. Absorbance is linearly related to concentrations by Beers Law, and this test detects phosphate concentrations of 5 to 1300 µg/L with a cuvette pathlength of l cm.
It is important to have an appropriately defined phosphorus detection limit. For example, a TP detection limit of 50 µg/L will not be adequate for a great deal of limnological efforts. Some phosphorus tests are designed for soils or sewage analysis and their minimum detection limits are inappropriate for limnological phosphorus analysis. As a matter of protocol, detection limits of 10 or 5 µg/L (or lower) will likely be necessary, to have adequate resolution for typical stream and lake monitoring. Using a rule of thumb that the detection level is about two times the standard deviation of replicate blanks, the lowest level of quantification is about five times this standard deviation. Hence, the level of quantification better defines the lower levels of identifiable TP concentrations, which occur with better (lower) detection levels.
Lower limits of detection can be obtained with a longer pathlength cell. Griesbach and Peters (1991) found that TP concentrations (after digestion) were relatively insensitive to even substantial changes in the concentration of the phosphorus reagents, and high precision was unnecessary when preparing the reagents. Results should be reported as the concentration of phosphorus, not phosphate, present in the water. Since orthophosphate is the form measured in the test, the results can be presented as PO4P.
Digestion Methods
As mentioned earlier, total phosphorus and total soluble phosphorus require a digestion step prior to the measurement of the orthophosphate form. There is no one perfect oxidant. The acid persulfate test that is often used is a compromise method that extracts most but not all phosphorus, but is a safer technique than others. Perchloric acid digestion, for example, will extract phosphorus from soils, but is a more complicated procedure and requires special facilities and safety precautions. Harwood et al. (1969) compared three different digestion methods (magnesium nitrate fusion, persulfate digestion, and peroxide digestion) on five types of liquid samples but found no significant differences in the resulting phosphorus concentrations.
Prepas and Rigler (1982) found that using persulfate alone produced less variable results than did perchloric acid or potassium persulfate with sulfuric acid (Jeffries et al. 1979). Standard Methods (1989) recommends the use of acid together with persulfate. It may be that the acid aids in the digestion of phosphorus from clays and other soil particles. Griesbach and Peters (1991), in an extensive examination of phosphorus techniques, found that the total phosphorus values resulting from varying the amount of persulfate added was not important within a range of 0.5 to 0.8 g persulfate per 40 ml sample. They suggested that weighing of each sample was not necessary; using a simple, volumetric scoop of persulfate was adequate. They also found that digestion time and whether the samples were autoclaved or boiled had no significant effect on the final phosphorus concentration. They suggest that boiling is more time-consuming than autoclaving but can be used if no autoclave is available.
Interferences
Several substances can interfere with the phosphorus analysis. Arsenate, which has been used to control algae and aquatic macrophytes, and high concentrations of silica can interfere. Where there is considerable humic color or clay turbidity, it may be necessary to run color/turbidity blanks for each sample. Keep a close watch on the physical characteristics of water samples. The phosphorus test is sensitive to trace amounts of contamination as is normally present in tap water, on fingers, in soap and some detergents, and in buffers or other reagents; be sure to clean glassware thoroughly, and observe maximum cleanliness in the field collection and in the laboratory. It is very important to run distilled water blanks and standards, keeping records of the absorbance in order to detect changes in distilled water quality or reagents.
Recommendations
Based on the theory discussed above and on the methods, observations, and comments by existing programs, the following recommendations are made:
- Based on ease of sampling, questions about storage, and utility of the information, total phosphorus should be the primary form of phosphorus measured in volunteer programs.
- Coordinators should first utilize surface (0.5 m) or an integrated epilimnetic samples in their programs, especially if chlorophyll and Secchi depth are to be measured at the same time. A second total phosphorus sample taken in the hypolimnion would add another dimension to the sampling program.
- The sampling program should have samples taken during spring turnover so that an estimate can be made of the total phosphorus content of the lake. An alternative is to take a detailed vertical profile during summer stratification.
- Although preservation of total samples is recommended, there are questions about the safety of having the volunteers handle strong acids. Coordinators should consider freezing the samples or having the digestion performed in the sample container itself.
- Volunteers should be given pre-cleaned, acid washed sample containers. Manipulation of the sample and the container by the volunteer should be minimized. Phosphorus differences can rapidly set up in a large container as particles settle. The volunteers should be instructed to shake the sample well before pouring. It would be better to pour directly from the water sampling device into the sample container rather than into a second container so that settling in the container and possible contamination is minimized.
- Soluble reactive phosphorus can also provide valuable information, but requires field filtration through a pre-cleaned membrane or glass fiber filter under clean circumstances. There are also questions of its stability with storage. If done, samples should be rapidly frozen and brought to the laboratory for analysis. Thought should be given to using a syringe filtration system so that the volunteers would not handle the filters.
- Research remains to be done as to the importance of the type and porosity of the filter on SRP analysis.
Literature Cited
Betz, C.R., P.J. Howard, and P.J. Anderson. 1990. Wisconsin’s expanded self-help lake monitoring program: Results of the 1990 pilot program expansion. Presented at the International Symposium of the North American Lake Management Society.
Canfield, D.E. Jr. 1991. Assessment of water quality in the lakes of north and central Florida. Project Completion Report.
Chamberlain, W.M. 1968. A preliminary investigation of the nature and importance of soluble organic phosphorus in the phosphorus cycle of lakes. Ph.D. Thesis, University of Toronto, Canada.
Downes, M.T. and H.W. Paerl. 1978. Separation of two dissolved reactive phosphorus fractions in lakewater. J. Fish. Res. Board Can. 35: 1636-1639.
Franko, D.A., and R.T. Heath. 1979. Functionally distinct classes of complex phosphorus compounds in lake water. Limnol. Oceanogr. 24: 463-473.
Griesbach, S.J. and R.H. Peters. 1991. The effects of analytical variations on estimates of phosphorus concentration in surface waters. Lake and Reservoir Management. 7: 97-106.
Harwood, J.E., R.A. Van Steederen, and A.L. Kahn. 1969. A comparison of some methods for total phosphate analyses. Water Res. 3: 425-432.
Jeffries, D.S., F.P. Diken, and D.E. Jones. 1979. Performance of the autoclave digestion method for total phosphorus analysis. Water Res. 13: 275-279.
Kitchell, Koonce, and Tennis. 1975. Phosphorus flux through fishes. Verh. Internat. Verein Limnol. 10: 2478-2484.
Nürnberg, G. and R.H. Peters. 1984. Biological availability of soluble reactive phosphorus in anoxic and oxic freshwaters. Can. J. Fish. Aquat. Sci. 41: 757-765.
Peters, R.H. 1986. The role of prediction in limnology. Limnol. Oceanogr. 31: 1143-1159.
Prepas, E.E. and F.H. Rigler. 1982. Improvements in quantifying the phosphorus concentration in lake water. Can. J. Fish. Aquat. Sci. 39: 822-829.
Redfield, A.C. 1958. The biological control of chemical factors in the environment. Amer. Sci. 46: 205-221.
Rigler, F.H. 1973. A dynamic view of the phosphorus cycle in lakes. In: E.J. Griffith, A. Beeton, J.M. Spencer, and D.T. Mitchell (Eds), Environmental Phosphorus Handbook. John Wiley & Sons.
Sakamoto, M. 1966. Primary production by phytoplankton community in some Japanese lakes and its dependence on lake depth. Arch. Hydrobiol. 62: 486-489.
Strickland, J.D.H. and T.T. Parsons. 1965. A manual of sea water analysis. Bull. 125. Fish. Res. Bd. Canada.
Vollenweider, R.A. 1976. Advances in defining critical loading levels for phosphorus in lake eutrophication. Mem. Ist. Ital. Idrobiol. 33: 53-83.